Duan, J., Huang, R. J.*, Li, Y. J., Chen, Q.*, Zheng, Y., Chen, Y., Lin, C., Ni, H., Wang, M., Ovadnevaite, J., Ceburnis, D., Chen, C., Worsnop, D. R., Hoffmann, T., O'Dowd, C., Cao, J. J.: Summertime and wintertime atmospheric processes of secondary aerosol in Beijing, Atmos. Chem. Phys., 20, 3793-3807, 2020.
Secondary aerosol constitutes a large fraction of fine particles in urban air of China. However, its formation mechanisms and atmospheric processes remain largely uncertain despite considerable study in recent years.
A research group led by Prof. HUANG Rujin from the Institute of Earth Environment (IEE) of the Chinese Academy of Sciences, used the Aerodyne quadrupole aerosol chemical speciation monitor (Q-ACSM) combined with PMF/ME-2 to elucidate the seasonal variations in fine-particle composition and secondary aerosol formation in Beijing between summer and winter.
The results suggest that photochemical oxidation was the major pathway for sulfate formation during summer, whereas aqueous-phase reaction became an important process for sulfate formation during winter. High concentrations of nitrate (17 % of the PM1 mass) were found during winter, explained by enhanced gas-to-particle partitioning at low temperature, while high nitrate concentrations (19 %) were also observed under the conditions of high relative humidity (RH) during summer, suggesting the contribution from aqueous-phase reactions (Figure 1). As for organic aerosol (OA) sources, secondary OA (SOA) dominated the OA mass (74 %) during summer, while the SOA contribution decreased to 39 % during winter due to enhanced primary emissions in the heating season. In terms of the SOA formation, photochemical oxidation perhaps played an important role for summertime oxygenated OA (OOA) formation and less-oxidized wintertime OOA (LO-OOA) formation. The wintertime more-oxidized OOA (MO-OOA) showed a good correlation with aerosol liquid water content (ALWC), indicating a more important contribution of aqueous-phase processing over photochemical production to MO-OOA (Figure 2 and 3).
This study, published in Atmospheric Chemistry and Physics, elucidated the seasonal difference in the formation and atmospheric processes of secondary aerosols between summer and winter under the influence of different weather conditions, which will help to understand the secondary aerosols formation in urban cities and formulate effective haze reduction measures.
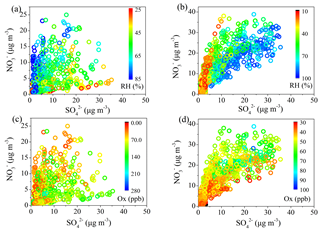
Fig. 1 Correlations between NO3- and SO42-, color coded with RH in summer (a) and winter (b), and correlations between NO3- and SO42-, color coded with Ox concentration in summer (c) and winter (d).
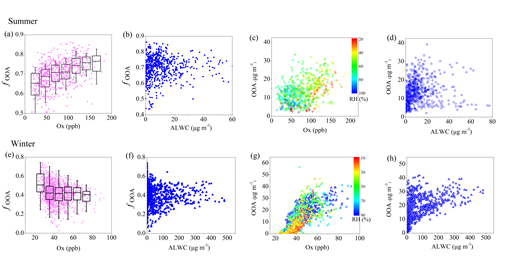
Fig. 2 Scatterplots of fOOA (mass fraction of OOA (related to OOA in summer and the total of LO-OOA C MO-OOA in winter) to OA) and mass concentration of OOA versus Ox and ALWC in summer (a–d) and winter (e–h).
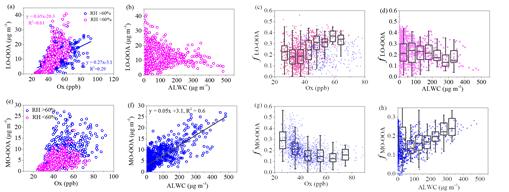
Fig.3 Relationship between LO-OOA or MO-OOA and Ox (a, e) and relationship between LO-OOA or MO-OOA and ALWC (b, f), as well as mass fractions of LO-OOA and MO-OOA as functions of Ox (c, g) and ALWC (d, h) in winter.

